About the Act on the Safety of Regenerative Medicine
The Regenerative Medicine Promotion (RMP) Act, which intends to enable people to receive safe regenerative medicine therapy in a timely manner, was promulgated in 2013 as a lawmaker-initiated legislation for comprehensively promoting policies for research, development, and practical applications of regenerative medicine. After discussions on specific policies for regenerative medicine based on the RMP Act, the Act on the Safety of Regenerative Medicine has been enacted and enforced since November 25, 2014.
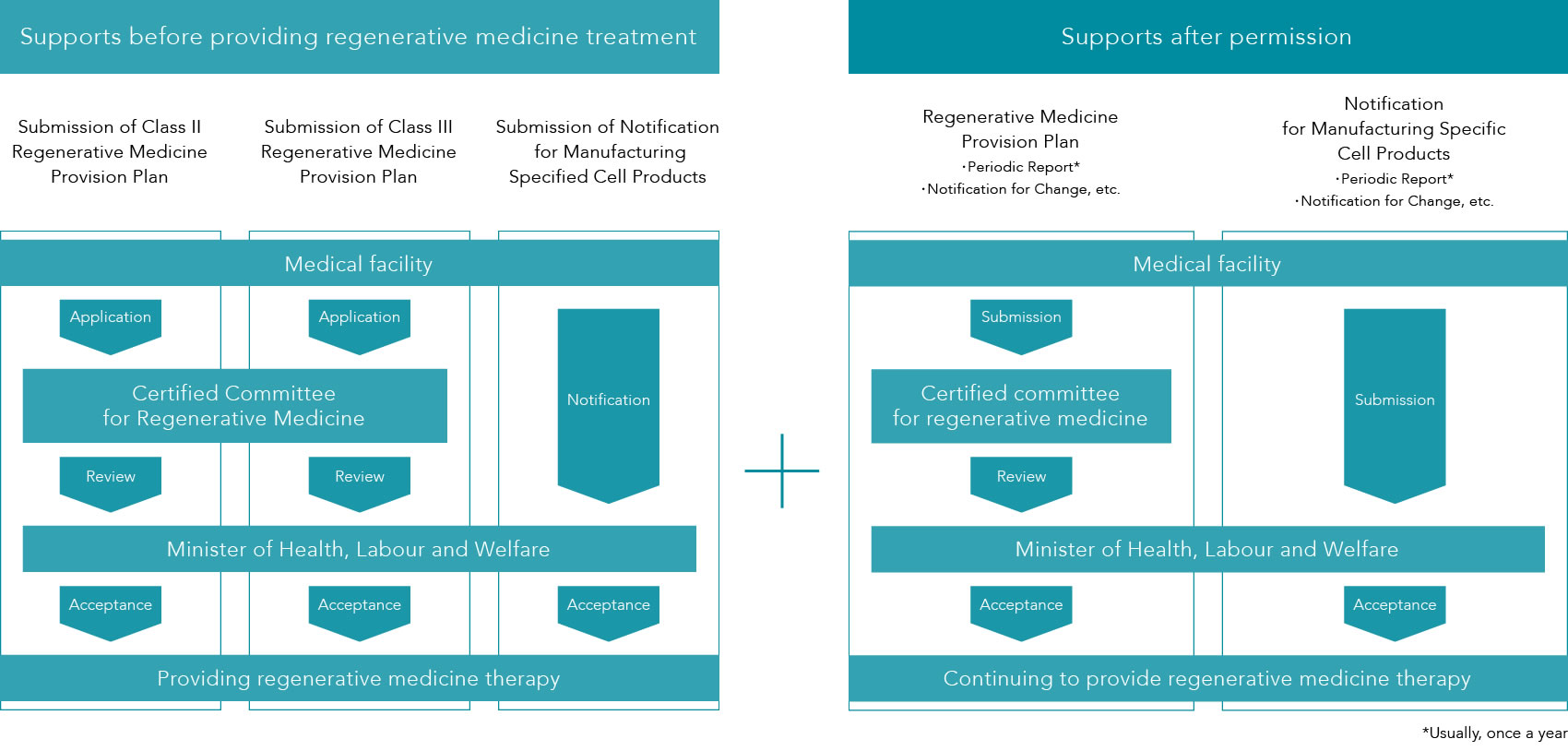
- The Act on the Safety of Regenerative Medicine (the Act) requires medical facilities to submit a regenerative medicine provision plan to the Minister of Health, Labour and Welfare according to the risk of the regenerative medicine therapy to be provided.
- Medical facilities manufacturing specific processed cells for therapy are required to submit a notification to the Minister of Health, Labour and Welfare.
- Failure to complete necessary regulatory procedures before providing regenerative medicine therapy is punishable under the Act.